Anthoceros, a genus of hornworts (a group of non-vascular embryophytes) in the order Anthocerotales, is widely distributed, mainly in tropical and temperate regions of the world.
- It belongs to the family Anthocerotaceae.
- The name Anthoceros means ‘flower horn‘, which refers to the characteristic horn-shaped sporophytes.
- Both primitive and advanced characteristics can be observed in Anthoceros.
Distribution of Anthoceros
- Anthoceros is the largest genus in the family Anthocerotaceae.
- This genus is represented by about 200 species.
- Anthoceros is present all over the world. It can be found mostly in temperate and tropical regions.
- All the species are terrestrial and grow in moist and shady places such as ditches, clay banks, hill slopes, or in the crevices of rock.
- Some species occur on decaying wood.
- Some of the common Anthoceros species are Anthoceros crispulus, A. erectus, A. himalayansis, A. chambenis, A. assamicus, A. dixitti, A. sahyadrensis, etc.
Gametophyte of Anthoceros
The gametophyte of Anthoceros is a small prostrate thallus. It is the dominant phase of the life cycle.

External Morphology of the Thallus
- The plant body of Anthoceros is gametophytic.
- The gametophyte is a small, prostrate, dorsiventral thallus.
- It is yellowish-green or dark green in colour.
- The thallus is generally dichotomously lobed (e.g., A. fusiformis), but in a few species it is sub-orbicular (e.g., A. crispulus).
- The lobes may be divided and have a wavey margin.
- Due to dichotomously branching, the thallus usually appears as an orbicular or semi-orbicular rosette form.
- Sometimes the thallus is raised on a thick ascending stalk-like structure (e.g., A. erectus) or bilobed (e.g., A. himalayensis) or pinnately branched (e.g., A. hallii).
Dorsal Surface
- The dorsal surface of the Anthoceros thallus may be smooth (e.g., A. laevis), velvet-like (e.g., A. crispulus), or rough with ridges (e.g., A. fusiformis).
- In A. crispulus, many lobed lamellae are present on the dorsal surface.
- The middle part of the thallus is several layers thick and without a distinct midrib.
- In some species, the midrib is absent.
- The mature Anthoceros gametophyte bears horn-like sporophytes on its dorsal surface.
Ventral Surface
- The ventral surface of the Anthoceros thallus bears numerous unicellular, smooth-walled rhizoids.
- The main functions of rhizoids are to attach the thallus to the substratum and to absorb water and mineral nutrients from the soil.
- Scales, tuberculate rhizoids, or mucilage hairs are absent on the ventral surface.
- Many small, rounded, thickened bluish green spots called mucilaginous cavities are present on the ventral surface.
- These mucilage cavities are filled with colonies of a blue-green alga like Nostoc.
Internal Morphology of the Thallus
In a vertical cross-section, the Anthoceros thallus shows a very simple structure without any internal differentiation of tissues.
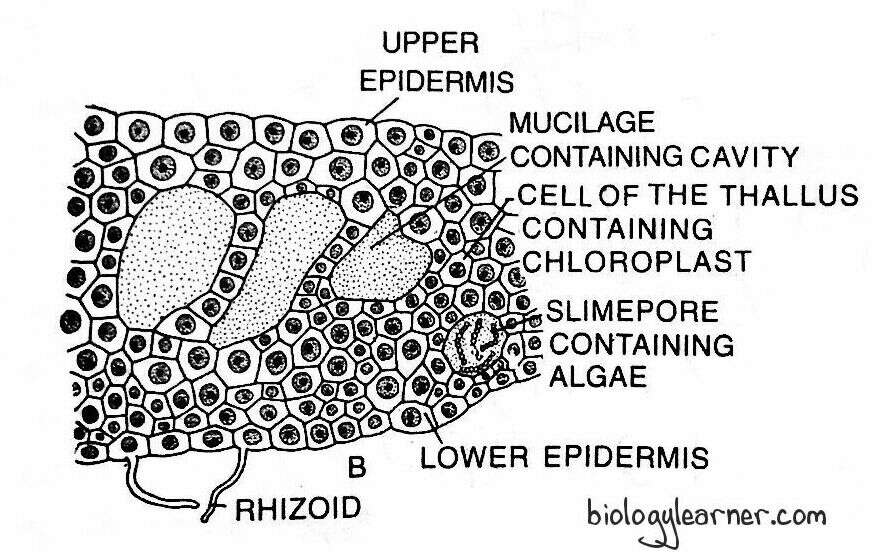
- The entire thallus is composed of uniform, thin-walled, parenchymatous cells except for the epidermis, which contains comparatively smaller cells.
- The thallus is several layers thick in the middle region.
- The thickness of the thallus varies in different species and is 6 to 8 cells thick in A. laevis, 8 to 10 cells thick in A. punctatus, and 30 to 40 cells thick in A. crispulus.
- The outermost layer of the thallus is the upper epidermis.
- The surface cells (epidermal cells) are regularly arranged. These cells have large chloroplasts and are smaller in size.
- Each cell of the thallus contains a single large chloroplast.
- The chloroplast is discoid or oval-shaped with a large pyrenoid (a characteristic feature of the class Anthocerotopsida) in the centre.
- The pyrenoid is actually formed by the accumulation of 25–50 disc-shaped or spindle-shaped bodies.
- Like green algae, starch grains are synthesised at the periphery of the pyrenoid in Anthoceros. This shows the algal ancestry of the Anthocerotales (order of Anthoceros).
- Also, the number of chloroplasts per cell varies in different species. In A. personi, each cell contains two chloroplasts, but in A. hallii, the number may be four to eight.
- A single nucleus is present in each cell very close to the chloroplast.
- There are no air chambers or pores in Anthoceros thallus.
- Instead, intercellular mucilage cavities are present on the ventral surface of the thallus. These are formed due to the breakdown of the cells (schizogenously).
- In some species, such as A. laevis and A. himalayensis, the mucilage cavities are absent.
- The mucilage cavities open on the ventral surface through a narrow slit or pore called the slime pore.
- Each slime pore contains two bean-shaped guard cells with thin walls.
- The guard cells are non-functional. They do not control the size of the pores. As a result, the pores remain completely open.
- Nostoc, a blue-green alga, invades these mucilage cavities through the slime pores and forms a colony in these cavities.
- The cavities containing Nostoc colonies can be seen to the naked eye as small, deep blue-green spherical spots on the ventral surface of the thallus.
- The lowermost cell layer of the thallus is the lower epidermis.
- A few cells of the lower epidermis extend to develop the smooth-walled rhizoids.
Reproduction in Anthoceros
Like Marchantia, Anthoceros also reproduces by both vegetative and sexual methods.
Vegetative Reproduction
Vegetative reproduction in Anthoceros occurs by the following methods:
Fragmentation
- Fragmentation is less common in Anthoceros than in liverworts.
- In this method, the progressive death and decay of the older basal part of the thallus (due to ageing or drought) reaches the place of dichotomy, and the lobes of the thallus get separated.
- Each separated lobe grows independently by apical growth and develops into a new thallus.
Gemmae
- In some species of Anthoceros, like A. propaguliferus, A. formosae, and A. glandulosus, many multicellular, short-stalked structures known as gemmae develop along the margins of the upper surface of the thallus.
- Sometimes gemmae develop mucilage pores.
- The gemmae detach from the parent thallus and grow into new plants.
Tubers
- In many species (e.g., A. tuberosus, A. halli, A. laevis, and A. pearsoni), under unfavourable conditions such as prolonged drought, the marginal patches of the thallus tissue get thickened to form the perennating tubers.
- The position of these perennating tubers varies in different species. They may develop along the margins of the thallus (e.g., A. pearsoni, A. hallii) or behind the growing points (e.g., A. laevis).
- In A. himalayensis, the tubers are stalked and grow along the margins of the thallus on the ventral surface.
- The tubers are protected from desiccation by 2 or 3 resistant layers of cork cells.
- The cork cells enclose the inner cells that store starch grains, aleurone granules, and oil globules.
- The perennating tubers can easily survive a period of drought and germinate into new thalli in a favourable season.
Persistent Growing Apices
- During the prolonged dry summer or towards the end of the growing season, the plants dry out.
- Only the growing apices with a little of the adjacent tissue survive.
- The apices remain dormant during the dry season.
- With the onset of favourable conditions, they grow into new thalli, e.g., A. pearsoni, and A. fusiformis.
Apospory
- In a few species of Anthoceros (e.g. A. laevis), the unspecialised cells of the various parts of the sporophyte, such as the intercalary meristematic zone, sub-epidermal and sporogenous tissue of the capsule, give rise to the gametophytic thallus.
- This phenomenon is known as apospory (development of the gametophyte from the sporophyte without the formation of spores; Lang, 1901; Schwarzenbach, 1926).
- These gametophytic thalli are diploid and sterile.
Sexual Reproduction
Sexual reproduction in Anthoceros is of the oogamous type. The gametes are developed in highly specialised reproductive organs. The male gametes (antherozoids) are produced in the antheridium (male reproductive organ), and the female gamete (egg) is formed in the archegonium (female reproductive organ).
Most of the Anthoceros species are monoecious (homothallic), i.e., antheridia and archegonia are produced in the same thallus (e.g., A. fusiformis, A. crispulus, A. himalayensis, A. gollani, A. longii, and A. punctatus). Some are dioecious (heterothallic), i.e., antheridia and archegonia are developed in the different thalli (e.g., A. hallii, A. pearsoni, A. erectus, A. laevis, and A. chambensis).
Monoecious species are protandrous, i.e., the antheridia mature before the archegonia. The formation of archegonia begins when antherozoids are mature.
The sex organs (antheridia and archegonia) are deeply embedded in the dorsal surface of the thallus.
Antheridium
The male reproductive organ of Anthoceros is the antheridium. The antheridium develops singly or in groups on the dorsal surface of the thallus.
Development of Antheridium
- In Anthoceros, the development of the antheridium begins from a single superficial dorsal cell of the thallus.
- The superficial dorsal cell is situated close to the growing apex. It usually divides by a periclinal wall into an outer roof initial and an inner antheridial initial.
- Unlike the class Hepaticopsida (e.g., Riccia), the antheridium of Anthoceros develops from the hypodermal cell (inner cell).
- Therefore, the antheridial initial is hypodermal rather than superficial, and the antheridium is endogenous in origin.
- Immediately after the division, a space is developed between the antheridial initial and the roof initial. This space, or cavity, is filled with mucilage.
- Meanwhile, the cavity enlarges and forms an antheridial chamber. The antheridial initial is pushed towards the base of the chamber.
- The roof initial first divides periclinally, followed by many anticlinal divisions to form a two-layered roof of the antheridial chamber.
- The antheridial initial may develop into a single antheridium (e.g., A. pearsoni) or it may divide vertically to form several antheridia (e.g., 7 in A. crispulus, 22 in A. erectus, and 30 in A. gemmulosus).
- Sometimes, younger antheridia also develop from the base of the older antheridium, resulting in the formation of a cluster of antheridia of different ages in a single antheridial cavity.
- Just before the division, the antheridial initial becomes almost superficial. It divides by two successive vertical divisions at right angles to each other and forms four cells.
- All four cells divide by a transverse division, resulting in eight cells, arranged in two tiers of four cells each.
- The lower stalk cells undergo a few further divisions to form the stalk of the antheridium.
- The cells of the lower tier are called stalk cells, while the cells of the upper tier are called antheridial cells.
- The upper antheridial cells divide by transverse division to form an eight-celled structure or octant stage.
- Periclinal division now appears in each cell of the octant, and there is the formation of eight outer primary jacket cells and eight inner primary androgennial cells.
- The primary jacket cells undergo several anticlinal divisions and form a single layer of the antheridial jacket.
- The primary androgonial cells undergo further divisions (transversely and vertically), resulting in the formation of a mass of small cubical androgonial cells.
- The last generation of the androgonial cells gives rise to androcyte mother cells (sperm mother cells or spermatocytes).
- Each androcyte mother cell undergoes a diagonal mitotic division and forms two triangular cells called androcytes or spermatids.
- The androcytes finally metamorphose into biflagellate antherozoids.

Structure of Antheridium
The mature antheridium is an orange-coloured elongated structure. It has a pouch-like or pear-shaped body with a multicellular stalk.

The stalk may be slender (as in A. punctatus and A. erectus) or more massive (as in A. laevis). It is composed of four or more rows of cells and attaches the antheridium to the base of the antheridial chamber.

In the same antheridial chamber, a single or cluster of two to four or more antheridia can be present. The body of the antheridium is encircled by a sterile jacket layer. The single-layered sterile jacket surrounds a number of androcytes, which metamorphose into antherozoids or sperm.
In some species, such as A. erectus and A. punctatus, the jacket layer is composed of four tiers of cells. The cells of the lower three tiers are elongated and rectangular. The cells of the uppermost tier are triangular with a narrow end towards the apex. In A. himalayensis and A. laevis, the jacket is formed of many relatively smaller and less regularly arranged cells.
Each cell in the jacket layer consists of plastids. As these plastids mature, their colour changes from green to red to bright orange. Therefore, young antheridia are green, and mature ones turn bright orange or reddish.
Antherozoids
The antherozoid is unicellular, uninucleate, bi-flagellated, and has a linear body. According to Proskaeur (1948), the bodies of antherozoids show some degree of residual curvature.
The pair of flagella are present at the anterior end of the antherozoid and are almost the same length as the body.
Dehiscence of Antheridium
The mature antheridium dehisces with the help of water. As the antheridia mature, the roof of the antheridial chamber ruptures irregularly, exposing the antheridia. The antheridia absorb water, and an aperture is formed by rupturing at the distal end of the antheridium.
A large number of antherozoids come out through the aperture. With the help of flagella, the liberated antherozoids swim in the film of water to reach the mature archegonia.
Archegonium
The female reproductive organ of the Anthoceros is the archegonium. The archegonium develops on the dorsal surface of the thallus close to the growing point. Due to the lack of a stalk, it is embedded in the thallus and is in direct contact with the vegetative cells.
Development of Archegonium
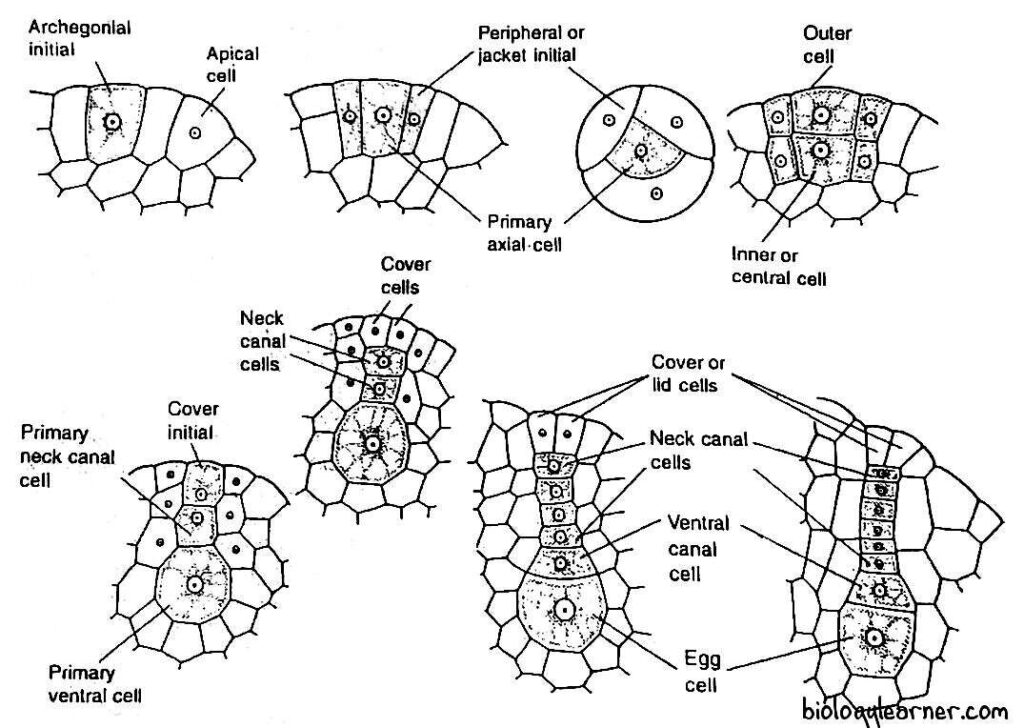
- The archegonium of Anthoceros develops from a single superficial cell on the dorsal surface of the thallus in an acropetal manner, called the archegonial initial.
- The superficial cell has dense protoplasm and lies close to the apical growing point.
- In monoecious species (e.g., A. gollani, A. punctatus, and A. fusiformis), the archegonia are formed after the antheridia develop in the same thallus.
- The archegonial initial divides transversely to form an outer primary archegonial cell and an inner primary stalk cell (e.g., A. gemmulosus, A. crispulus). But in A. erectus, it may directly function as a primary archegonial cell (Mehra and Hanson, 1953).
- The primary archegonial cell divides by three successive intersecting walls or periclinal vertical walls, resulting in the formation of three sterile peripheral initials or jacket initials and a fourth fertile median cell, the primary axial cell.
- Peripheral initials or jacket initials divide transversely to form two tiers of three cells each.
- The cells of the upper tier divide by an anticlinal division and form six cells. These cells divide by transverse division to form a jacket of six rows of sterile neck cells.
- The cells of the lower tier divide by transverse and vertical divisions to form a venter wall.
- Simultaneously, the primary axial cell divides transversely to form a lower central cell and an upper cell.
- The upper cell divides into a terminal cover initial and a lower primary neck canal cell.
- The lower central cell directly functions as the primary venter cell, and it divides an unequal transverse division to form an upper small venter canal cell and a lower large egg.
- The primary neck canal cell undergoes a series of transverse divisions to form a vertical row of four to six or more neck canal cells.
- The cover initial divides by one to two vertical divisions at right angles to each other, forming two to four rosette-like cover cells at the tip of the neck.
Structure of Archegonium
The mature archegonium remains completely embedded in the dorsal surface of the thallus in direct contact with the surrounding vegetative cells.
Each archegonium consists of two to four cover cells, a vertical row of four to six neck canal cells, a venter canal cell, and an egg.

The cover cells are present at the tip of the archegonium and protrude slightly above the general surface of the thallus. They are usually associated with the funnel-shaped mass of mucilage called the mucilage mound.
There is no sterile jacket layer or the jacket layer is not distinct from the other vegetative cells.
Fertilization
Like other bryophytes, water is essential for fertilization in Anthoceros.
In the mature archegonium, the neck canal cells and the single ventral canal cell disintegrate to form a mucilaginous mass. The mucilage absorbs water, swells, and comes out of the archegonial neck by forcing upon the cover cells, forming an open passage down to the egg.

The mucilage mass contains chemical substances (chemical proteins or potassium salts) that attract the antherozoids.
A number of antherozoids caught in the mucilage enter the archegonial neck due to the chemotactic response and reach up to the egg.
Finally, one antherozoid fertilises the egg to form a diploid zygote or oospore. The zygote enlarges in size and fills the cavity of the venter.
The gametophytic phase ends after fertilisation.
Sporophyte of Anthoceros
The sporophytic phase in Anthoceros is the direct result of the sexual process and starts with the formation of the diploid zygote or oospore after fertilization. The zygote is the first cell of the sporophyte.
After fertilization, the diploid zygote or oospore immediately secretes an outer cellulose wall around itself. It increases in size and almost completely fills the cavity of the venter in the archegonium.
Due to its structure and degree of independence, the sporophyte of the Anthoceros occupies a significant position in the evolutionary sequence of plants.
Development of Sporophyte
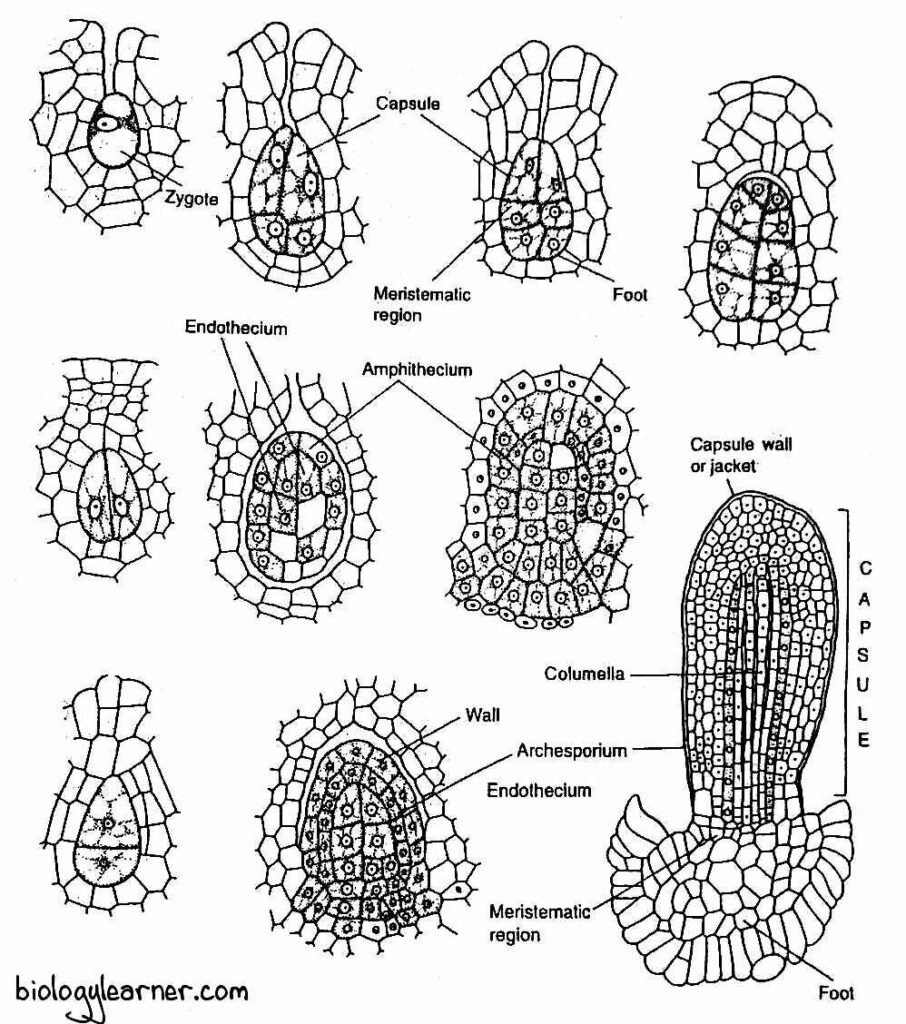
- The zygote divides first by a vertical division, the exact opposite of other bryophytes (in other Bryophytes except for hornworts, the first division of the zygote is transverse).
- The subsequent division is at a right angle to the first (dividing transversely) and forms a four-cell stage, the quadrant.
- The upper two cells are generally longer than the lower two in the quadrant.
- Each of the four cells in the quadrant is then divided by a vertical wall at a right angle to the preceding one.
- As a result, the embryo develops into an eight-celled structure or octant stage. The eight cells are arranged into two tiers of four cells each.
- Further development of the sporophyte varies in different species of Anthoceros. In A. erectus, the lower tier of four cells of the octant forms the foot, while the seta and capsule are developed from the upper tier of four cells.
- In most of the species, such as A. pearsoni, A. himalayensis, and A. fusiformis, the upper tier of four cells divides transversely to form three tiers of four cells each.
- The lowermost tier forms the foot, the middle tier forms the meristematic zone or intermediate zone, and the uppermost tier gives rise to the capsule.
- The lower tier of four cells divides by several irregular divisions and forms a sterile bulbous foot. The foot is composed of many parenchymatous cells.
- In some species, like A. himalayensis and A. laevis, the superficial cells (lowermost cells) of the foot develop into haustoria-like or rhizoid-like projections, while in A. punctatus, the superficial cells form a palisade layer of cells.
- The rhizoids increase the absorptive surface for the absorption of nutrients from the gametophyte.
- The upper tier of four cells divides and its lower cells form an intercalary meristematic tissue.
- The upper cells of the upper tier divide periclinally (parallel with the surface) into an outer layer of amphithecium and a central mass of cells called endothecium.
- The cells of the endothecium form the columella, which is the central sterile part of the capsule.
- The columella is initially composed of four vertical rows of cells, but as the sporophyte matures, the number of rows of cells in the columella increases to sixteen.
- The amphithecium divides by a periclinal wall into an outer sterile layer of jacket initials or wall initials and an inner fertile layer of sporogenous cells.
- The cells of the jacket initials divide by anticlinally and periclinally to form a four to six-layered protective sterile jacket or capsule wall.
- The outermost layer of the jacket is called the epidermis.
- The epidermis is characterised by the presence of stomata.
- The cells of the inner layers of the jacket are parenchymatous and contain chloroplasts.
- The sporogenous cells later form the archesporium.
- The archesporium may extend to four layers in thickness. In A. erectus, it is single-layered and it becomes two-layered in A. pearsoni or two to four-layered in A. hallii.
- As the sporogonium advances towards maturity, the cells of the archesporium divide and re-divide several times to form two types of cells: spore mother cells and sterile cells (elater mother cells). These cells are arranged in an alternate manner, one above the other.
- Each of the spore mother cells is large, oval or spherical, with dense cytoplasm and a large nucleus. It is diploid and divides reductionally (meiotic division) to form haploid spore tetrads (four spores arranged tetrahedrally).
- The sterile elater mother cells are diploid, elliptical with small nuclei. These cells are smaller than spore mother cells and divide mitotically to produce four-celled elaters.
- The four cells of the elaters may remain attached to each other or may split into one-celled, two-celled, or three-celled units.
- The broken units are known as pseudoelaters, which do not have any thickened bands (the elaters are without any thickening bands and, therefore, are called “pseudo elaters”). So the pseudoelaters may be simple or branched in Anthoceros.
- By the activity of the meristematic zone (base of the capsule), various tissues continuously produce new cells so that the sporophyte becomes elongated.
- The new cells of the capsule gradually mature into the different regions, i.e., the columella, the sporogenous layer, and the jacket of the mature capsule. Thus, the sporophyte remains active for a long time.
- The developing sporophyte of Anthoceros is surrounded by a fleshy protective covering or sheath called the involucre.
- The upper part of the involucre is derived from the venter of the archegonium and the lower part from the cells of the gametophytic thallus.
- The sporophyte is completely surrounded by involucre in its early stages. As the sporophyte elongates, the involucre is infiltrated through the apex.
- A portion of the involucre remains at the apex of the sporophyte (it resembles the calyptra of mosses).
- The lower portion develops a collar around the base of the sporophyte.The collar protects the weak intercalary meristem and helps to retain moisture.
Structure of Sporophyte
The mature sporophyte of Anthoceros is an elongated structure. They usually grow in clusters from the dorsal surface of the thallus.
The sporophyte is about 2 to 15 cm long and appears like a horn or bristle; hence, the species are commonly called hornworts. The young sporophyte is green in colour. But as the sporophyte matures, it gradually turns green to black or dark yellow from the apex to the base.
The sporophyte of the Anthoceros can be differentiated into three regions: the foot, the intercalary or intermediate zone, and the capsule. Seta is absent (the intercalary meristematic zone is present instead of the sita).

Foot
The foot is the basal part of the sporophyte. It is a rounded, bulbous structure composed of many parenchymatous cells.
The foot is deeply embedded in the tissue of the gametophytic thallus. The lowermost cells of the foot produce short, tubular rhizoid-like projections that function as haustorium. They absorb nutrients and water from the gametophyte for the developing sporophyte.
Intercalary or Intermediate Meristematic Zone
Between the foot and the capsule, a short intermediate or intercalary zone is present instead of seta. This zone is made up of meristematic cells.
The meristematic cells constantly add new cells to the capsule at its base. Thus, the capsule is always at different stages of development from base upwards in the sporophyte of Anthoceros. It is in the embryonic stage at the base and very mature at the apex.
The meristem enables the capsule to grow for a long period and produce spores.

Capsule
The capsule forms the major and most conspicuous part of the sporophyte. It is a smooth, slender, erect cylindrical structure that slightly tapers at the apex.
The capsule can be differentiated internally into columella, archesporium (sporogenous tissue), and capsule wall.
Columella
The columella is the central sterile part of the capsule. It extends nearly to the apex of the sporophyte and originates from the endothecium.
In a young sporophyte, the columella is made up of four vertical rows of cells. But in a mature sporophyte, it consists of 16 vertical rows of cells. The cells are narrow and vertically elongated.
In the transverse section, the columella appears as a solid square.
The columella provides mechanical support to the long capsule. It also helps in the dispersal of spores and is involved in the conduction of water and mineral nutrients.
Archesporium or Sporogenous Tissue
The archesporium, or sporogenous tissue, is situated in between the columella and the capsule wall. It is derived from amphithecium.
In some species of Anthoceros, such as A. erectus, A. crenatifrons, and A. hawaiensis, the archesporium may remain single-layered throughout its further development. However, in A. himalayensis and A. pearsoni, it becomes two-layered thick a little above the base of the capsule. In A. hallii, it may even become two to four layers thick.
Over the top of the columella, the archesporium extends like a dome. The cells of the archesporium can easily be distinguished from the sterile cells of the columella due to the presence of their dense protoplasm.
In the upper part of the capsule, the sporogenous tissue (archesporium) is gradually differentiated into spherical or oval fertile spore mother cells and slender sterile pseudoelater mother cells.
The sterile cells (pseudoelater mother cells) increase in length and join end to end, forming simple or branched one to four-celled pseudoelaters. The cells of pseudoelater are smooth-walled and more or less elongated.
The spiral bands or thickenings are absent in pseudoelaters in all the Anthoceros species. However, in A. physocladus, they have long and thick walls with an extremely reduced lumen (Pande, 1960). In some species, the secondary walls of pseudoelaters possess helical thickenings (Proskauer, 1960).
Pseudoelaters are hygroscopic in nature. They help the spores to disperse.
Each spore mother cell forms four haploid spores arranged tetrahedrally.
Capsule Wall
The wall or jacket of the capsule is four to six layers thick and composed of parenchymatous cells. It originates from amphithecium.
The outermost layer of the capsule wall is the epidermis. The cells of the epidermis are narrow, and vertically elongated, with their outer walls heavily cutinised.
The epidermis is interrupted by the presence of stomata. The stomata are oriented vertically with respect to the axis of the sporophyte and are widely separated from each other. Each of the stomata contains a pore surrounded by two guard cells.
Below the epidermal layer of the capsule wall, the cells are chlorenchymatous and have intercellular spaces.
There are two large chloroplasts present in each cell. Thus, the sporophyte is actively photosynthetic in function. Hence, it partially depends on the gametophyte for the supply of water and minerals.
Spore
The spores are haploid, uninucleate, and tetrahedral in shape. They are very small and are surrounded by thick wall layers.
The wall is two-layered; the outermost exospore or exine and the innermost endospore or intine. The outermost exospore is thick and ornamented, while the innermost endospore is thin. Exospore varies in colour from dark brown to black (e.g., A. punctatus) or yellowish (e.g., A. laevis).
Inside the wall layers, the spore consists of a single nucleus, colourless plastids, reserve food materials, and oil globules.
Dehiscence of the Capsule and Dispersal of Spores
The capsule dehisces basipetally (from apex to base). As the capsule matures, its apex (tip portion) becomes grey, brown, or black.
The tip of the capsule loses water, shrinks, and consequently, vertical lines of dehiscence appear in the capsule wall.
The capsule usually dehisces along two to four longitudinal lines. Due to the drying of the capsule wall, the narrow slits appear along the line of dehiscence (shallow grooves), which gradually widens downwards. In A. crispulus, the capsule splits first along one line of dehiscence and is followed by splitting along the other line of dehiscence. As a result, the capsule wall slits into two to four valves.
As the upper part of the capsule dries up, the valves separate and start to twist longitudinally. Subsequently, the pseudoelaters also dry out, twist, and help to loosen the spore mass. The twisting valves thus expose the pseudoelaters and spores.
Eventually, the spores are shed from the exposed capsule by the hygroscopic movement of the pseudoelaters, valves, and columella. The liberated spores are dispersed to a distance by air currents.
Germination of Spores and Formation of the Young Gametophyte
The spore is the first cell of the gametophytic generation.
Each spore germinates upon falling on a suitable moist substratum, either immediately (e.g., A. punctatus and A. erectus; Mehra and Kachroo, 1962) or after a resting period of a few weeks to a few months (e.g., A. fusiformis; Campbell, 1928).
Under favourable conditions, at the time of germination, the spore absorbs moisture from the substratum and increases considerably in size.
The exospore ruptures at the triradiate mark and the endospore comes out as a tubular germ tube called the germinal. The contents of the spore move to the germinal tube and the colourless plastids become green.
The tip of the germinal tube is now divided by a transverse wall to form a small distal cell. It is again divided by transversely, which is parallel to the first one.
These two cells undergo two vertical divisions at right angles to one another, forming two tiers of four cells each. This eight-cell structure is called the octant stage or sporeling.
The distal tier of four cells of the octant functions as an apical cell which divides to form a new gametophytic thallus. Along with these divisions, the first rhizoid develops as an elongation of any cell of the young thallus.
Evolutionary Significance of Anthoceros Sporophyte
In Anthoceros, there is a remarkable association of a primitive gametophyte with a highly developed sporophyte. The sporophyte is distinctly different from the sporophyte of the other bryophytes and is considered an advanced type.
The noteworthy features that also indicate evolutionary trends found in Anthoceros sporophyte are:
- The beginning of physiologically independent sporophyte with the development of photosynthetic green tissue.
- The epidermis of the capsule wall is interrupted by stomata similar to those of the higher plants.
- Establishment of a sterile axis by transforming the sporogenous tissue from the central portion (i.e., endothecium) to the outer region (i.e., amphithecium) of the capsule. The chance suggests the beginning of a region for the development of vascular tissue and superficial sporangia.
- The arrangement of fertile and sterile tissues into alternate bands in the Anthoceros capsule is the basis for the origin and evolution of leaves and sporangia in the pteridophytes (theory of origin of the strobilus).
- The erect capsule (body of the sporophyte) helps in the efficient dispersal of spores.
- In exceptionally favorable conditions for growth, the capsule of A. fusiformis can reach a length of 6 inches, and there was evidence that the surface of the foot makes direct contact with the soil due to the disorganization of the adjacent thallus tissue.
Taxonomic Position of Anthoceros
| Division: | Bryophyta |
| Class: | Anthocerotopsida |
| Order: | Anthocerotales |
| Family: | Anthocerotaceae |
| Genus: | Anthoceros |
References
- Rashid, A. (1998). An Introduction to Bryophyta. Vikas Publishing House.
- Vashishta, B. R. (2005). Bryophytes. S .Chand Publishing Company.
- Frangedakis, E., Shimamura, M., Villarreal, J. C., Li, F., Tomaselli, M., Waller, M., Sakakibara, K., Renzaglia, K. S., & Szövényi, P. (2020, September 15). The hornworts: morphology, evolution and development. New Phytologist, 229(2), 735–754. https://doi.org/10.1111/nph.16874
- Chopra, R. N. (2005). Biology of Bryophytes. New Age International.
