Sterilization is the complete eradication of microorganisms (fungi, bacteria, and viruses) present on the surface of any material.
The microorganisms can be removed or destroyed from the definite materials (seeds, leaves, root pieces, stem pieces) by using sterilization techniques.
Objective
Performing different methods of sterilization used in biotechnology.

Principle of Sterilization Techniques
Maintaining a sterile environment during the transfer, or culturing of cells or tissues of microbes, plants, and animal cells is most important.
The concept of sterilization, for making the materials free from any type of contamination was given by Louis Pasteur. Thus sterilization is a process of making an article, surface, or medium free from any type of microorganisms that contaminate the object and provide unwanted results.
However, sterilization is one of the most important steps for the cultivation, isolation, and study of purified cells or tissues in the laboratory.
The other important things to be sterilized are the surgical tools, culture vessels, nutrient media, and plant materials. Some other methods used to make these sterile are disinfection and incineration.
- Disinfection: Disinfection is a similar process of killing harmful organisms, especially the objects but not the culture media. Disinfection of tabletops, equipment, and other surfaces is usually done by using glycolic acid compounds, carbolic acid, formaldehyde, ethanol, etc.
- Incineration: It is a process of killing microorganisms by using a flame, therefore, it is called flame sterilization. It is done by keeping the inoculation needle over the flame of the Bunsen burner till it becomes red hot. Thus, the microorganisms present on the surface of the needle are destroyed.

Physical Methods of Sterilization
There are several physical methods of sterilization of materials and objects. These are the following:
Moist Heat
Culture media (liquid and agar), water, glassware, surgical blades, and scalpels are sterilized by using moist heat (steam under pressure). It is done by using an autoclave, and also by a pressure cooker (used for cooking pressure at home).
Dry Heat
Dry heat sterilization is carried out by using a hot air oven. Glassware, glass syringe, forceps, scalpel, pipettes, flasks metallic instruments, Petri dishes, etc. are sterilization in an oven at 150°C for 1 hour or 250°C for 30 minutes.
Radiation
Normally UV radiation is used in an inoculation chamber or laminar airflow. Expose the working area to UV radiation before 2 hours to start the work. The source of UV radiation is UV lamps or tubes enclosed in quartz because the glass will not transmit UV radiation.
Care should be taken not to see the UV radiation with naked eyes. Otherwise, any abnormality may occur in the eyes.
Membrane Filtration
Sterilization of heat-sensitive substances like enzymes, antibiotics, and amino acids could not be done by autoclaving because these may be denatured and rendered non-functional.
Hence, these are sterilization through various types of filters which may retain bacteria.
Millipore membrane filters are commonly used for this purpose. It is commercially available (Nalgene, Billipore, and Whatman in England) membrane filters. It is made up of cellulose acetate or cellulose nitrate that contains small-sized pores of varying diameters (0.22, 0.45, 0.5 μm). But membrane filter of 0.22 μm is generally preferred.
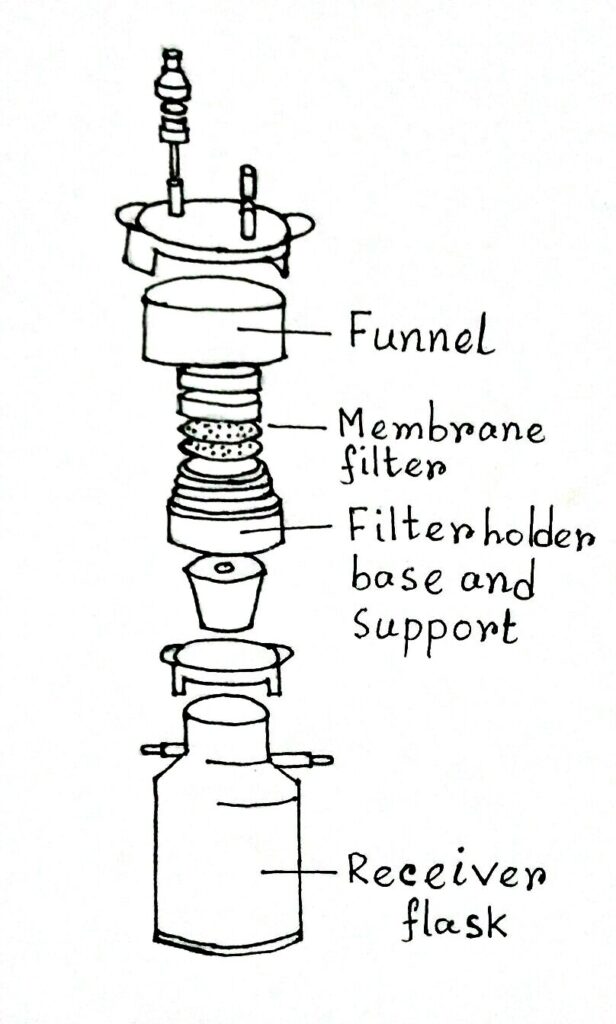
A Millipore membrane filter is placed inside the filtration assembly which is made up of autoclave plastic materials, stainless steel, or glass. The whole assembly containing millipore filter paper is sterilized by autoclaving before use.
In such cases, the solutions to be sterilized usually are passed through membrane filters by negative pressure applied through suction or centrifugal force. The filtrate so obtained is collected in a sterile container, and the filtrate becomes microbe-free.
Chemical Methods of Sterilization
There are several chemical methods of sterilization of materials and objects. These are the following:
Alcohol
Ethanol (70%) or propane (70%) is used to sterilize the working tabletop, inoculation chamber, hands, materials to be used, glass apparatus, etc.
Aldehyde
Generally, the laboratory or chamber is fumigated with formaldehyde when the number of contaminants gets increases.
Inorganic Chemicals
There are certain chemicals toxic to any organisms such as salts of copper, mercury, etc. HgCl2 solution (0.1%) is most commonly used as a disinfectant for seeds, explants, and plant materials. For the same purpose, other chemicals used are sodium hypochlorite (NaOCl) (10%).
The materials to be disinfected in the solution are kept in HgCl2 solution for 5-10 minutes (for naked hypochlorite). Soon take out the materials, transfer them into sterilized distilled water, and washed properly. Again repeat the process of washing 5-8 times to remove the traces of chemicals.
Requirements of Sterilization Techniques:
- Autoclave (for moist heat sterilization), laminar airflow (fitted with UV tube)
- Oven (for dry heat sterilization)
- Seed/leaves/root pieces/stem pieces of any plants.
- Spirit, Ethyl alcohol (70%), Mercuric chloride (0.1% HgCl2), Sodium
- hypochlorite/Calcium hypochlorite (10%) (for chemical sterilization)
- Vacuum pump, filtration unit, and Whitman filter paper (0.22 μm) (membrane filtration sterilization)
- Sterile distilled water, glassware, cotton plugs, Bunsen burner, absorbent cotton, plasticware, Petri dishes, etc.
- Surgical blades, holders, forceps, etc.
- Plant seedling (10 days old)
Procedures of Sterilization Techniques
Sterilization of Glassware and Objects
For complete sterilization suitable containers having glassware, glass syringe, forceps, surgical blades holder, needles, scalpels, and metal instruments are kept in an oven at 150°C for 1 hour or 250 °C for 30 minutes.
Sterilization of Nutrient Media
Culture media (broth or agar media), water, and other material (Millipore filter) are sterilized by an autoclave (high-pressure stem using moist heat) at 15 psi (pound per inch square) for 20-30 minutes which gives 121°C.
Glassware must be wrapped with aluminum foil and flasks containing nutrient medium, must be plugged with cotton, and then wrapped with aluminum foil. After sterilization, the materials should not be taken out immediately.
Then wait for 15-20 minutes to lower the temperature to about 60°C. After releasing the pressure, the material should be taken out.
Surface Sterilization
For surface sterilization of seeds and other parts of a plant (leaves, roots, bark), these are washed with tap water. Then it is dipped in disinfectant chemical solutions first in 70% Ethyl alcohol for 30 seconds, then in 10% sodium hypochlorite for about 10-20 minutes.
The time of sterilization varies with the materials used. The materials should be thoroughly washed (5-10 times) with double distilled water (5-8 times repeat this process). Sterilization procedures should be performed under aseptic conditions (laminar airflow).
Sometimes seeds are dipped only in 10% Sodium hypochlorite or Calcium hypochlorite for 5-10 minutes of sterilization without any other treatment.
Membrane Filtration Method
The membrane filtration method is used only for those chemicals which are heat sensitive or unstable at high temperatures (enzymes, antibiotics, vitamins, amino acids).
Commonly, the Whatman filter (0.22 μm) is used for sterilization. These substances are passed through ultra-filtration membrane filters using a vacuum pump.
Results of Sterilization Techniques
If cultural media are not autoclaved properly, they will be contaminated by microorganisms. Due to excess autoclaving, culture media turns into brown color.
Surface sterilization of seeds with chemicals for a long time affects the viability of seeds and other plant parts.
Precautions of Sterilization Techniques
- Before the operation of laminar flow, the working surface should be sterilized with 70% Ethyl alcohol and UV radiation for 30 minutes.
- Hands should be washed with 70% Ethyl alcohol before handling the plant materials.
- Surgical hand gloves should also be used while performing the experiments under aseptic conditions.

What a nice post