Protoplast is a plant cell (organized mass) devoid of the cell wall. Protoplasts are useful for the study of physiological problems of the cells and isolation of organelles and the study of the exogenously applied material on cell activity.
These are prepared from the plant tissues, callus, and cultures of suspensions.
Introduction
The protoplast is the organized mass that lies within the cell wall of the plant cell. When the plant tissue is cut into small-sized pieces and treated with pectinase enzyme, the cells are separated from the connective tissue by the enzymes. Cellulase and hemicellulase enzymes break down the cell wall leaving only the cell membrane. Consequently, protoplasts are liberated where cells lose their original shape and turn into round shapes. The osmotic potential of the cells is maintained by using mannitol or sorbitol in the digesting medium. This prevents the bursting and shriveling of the protoplasts.
When the protoplasts from the same species are kept in close contact in a proper buffer solution, they may fuse spontaneously. The frequency of fusion may be increased by using specific chemicals called fusogens (for example- sodium nitrate, polyethylene glycol, Ca2+, and high pH). The fusogens lower the surface charge and thus permit the protoplasts to come into close proximity for fusion.
Protoplast fusion is a useful tool for plant agriculturists to use in making crosses between sexually incompatible species. Through protoplast fusion, one can transfer cytoplasmic traits between the species (interspecific), within the species (intraspecific) and within the geneta (intergeneric).
Objective
Preparation of protoplasts from plant tissue and demonstration of somatic fusion.
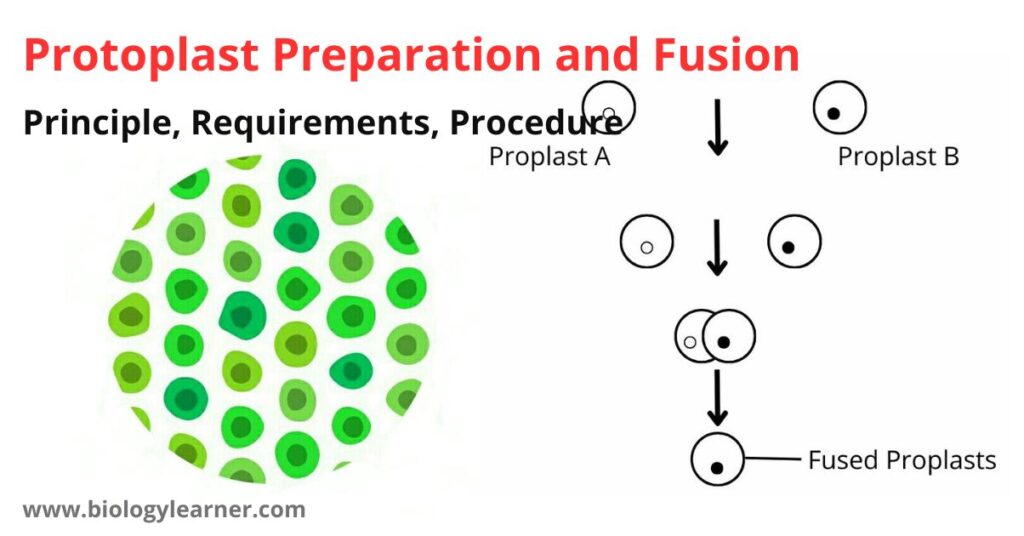
Principle of Protoplast Preparation and Fusion
When plant tissues are treated with the cell wall lysing enzymes and incubated properly, the plant tissues are digested by the enzymes, and protoplasts are released. In the presence of fusogen, these protoplasts can fuse with each other.
When the protoplasts are obtained from colored plant tissue, the protoplasts also appear colored and can easily be seen under the microscope.
When the protoplasts are obtained from two differently colored tissues, the fusion products can easily be seen and identified as homokaryon (fusion of protoplasts from the same tissue) or heterokaryon (fusion of protoplasts obtained from different plant tissue).
Requirements for Protoplast Preparation and Fusion
- Plant tissues such as petals of a flower, in vitro grown green tissue, young leaves, a microscope
- Pasteur pipettes, rubber bulbs, mannitol
- Macerozyme (a mixture of pectinase, cellulase, and hemicellulase) (2% in 0.6 M mannitol, pH 5.8)
- Polyethylene glycol (PEG) (5 g/10 ml)
Procedure of Protoplast Preparation and Fusion
Isolation of Protoplasts
- Collect healthy leaves or petals of colored flowers or in-vitro grown plant tissues.
- Peel off the epidermis and shred the leaf tissue (grown preferably under aseptic conditions) into small pieces. The colored petals of flowers (that have anthocyanin) can also be used.
- Put 200 mg of tissue in 2 ml of enzyme solution taken in a Petri dish.
- Cover the Petri dish and incubate for 4-6 hours at 30°C at 50 rpm. Observe the isolated protoplasts under a simple microscope.
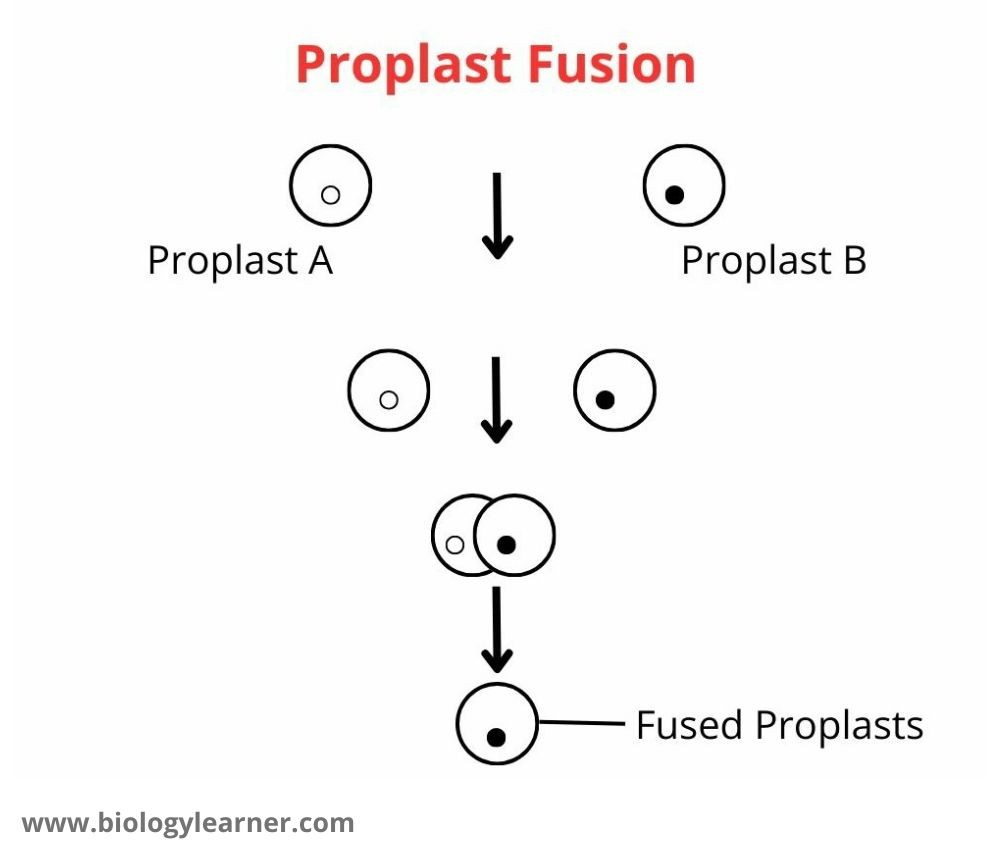
Fusion of Protoplasts
- Put one drop of suspension containing protoplasts and one drop of petal protoplasts on a slide.
- Add one drop of PEG in the center of the slide to allow the drops of protoplasts to come closer. Observe the slides under the microscope after 10 minutes.
Results
The isolated protoplasts appear as rounded structures of various sizes. The heterokaryon fusion products appear in different colors.
