Antibodies are the proteins produced by the B-lymphocytes in response to the antigen or foreign bodies.
- They are also known as immunoglobulins (Ig).
- They are found in the serum fraction of the blood and produced by plasma cells. Approximately 20% of the plasma protein is immunoglobulins.
- Antibodies can travel throughout the body to find and bind to their target antigens (foreign substances).
- The molecular weight of antibodies are generally ranges between 150000-900000 Da.
Structure of Antibody
IgG is the most studied immunoglobulin and serves as a model of the basic structural unit of all antibodies.
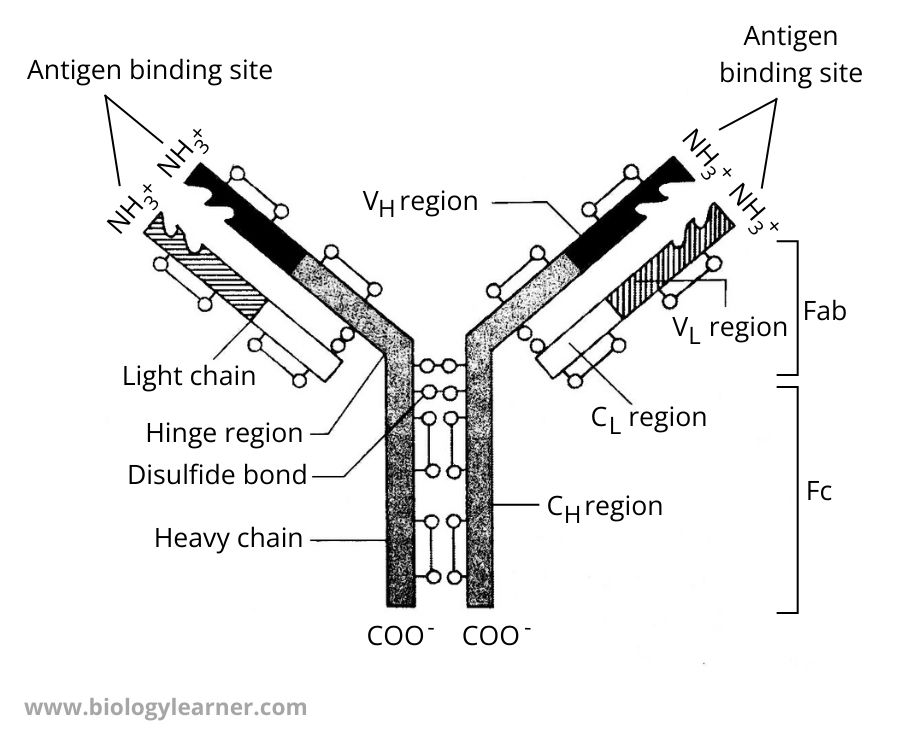
The molecule of IgG is Y-shaped and consists of 4-polypeptide chains; two identical heavy (H) chains and two identical light (L) chains which are connected by a disulfide bond.
Different parts of an antibody molecule are described below:
Heavy and Light Chains
- There are 4 peptide chains in an antibody molecule.
- Two long chains called heavy chains and two long chains called light chains.
- Each heavy chain contains more than 400 amino acids, while the light chain usually has 200 amino acids.
- In mammals, H chains are α, δ, ε, γ, and μ types, but L chains are two types known as lambda (λ) and kappa (κ).
Disulfide Bonds and Hinge Region
- Each light chain is linked to a heavy chain by a disulfide bond.
- There are also two disulfide bonds connecting the two heavy chains.
- This part of the antibody is called the hinge region, which functions in maintaining the flexibility of the antibody. Here, the arms of the antibody can bend and form a Y-shaped structure.
- There are also intrachain disulfide bonds in both light and heavy chains.
Constant and Variable Regions
- Intrachain disulfide bonds form loops.
- Each loop is called a domain.
- The light chain contains a single variable domain (VL)and a single constant domain (CL), while the heavy chain contains a single variable domain (VH) and three or four constant domains (CH).
- Constant domains from the constant region and variable domains form the variable region in each of the heavy and light chains.
Fragment Antigen Binding (Fab) and Fragment Crystalizable (Fc)
- Two identical fragments of the Y-shaped structure of the antibody molecule function for antigen binding sites. This is known as the fragment antigen binding (Fab).
- The antigen binding sites bind to specific antigens in a lock and key pattern and form an antigen-antibody complex.
- The tail section of the antibody is incapable of binding antigen and can be crystallized. This is called fragment crystallizable (Fc).
Types of Antibodies
There are different isotypes or classes of antibodies. On the basis of amino acid sequences in the heavy chain constant regions, antibodies are of five isotypes or classes. These are IgG, IgM, IgA, IgD, and IgE.
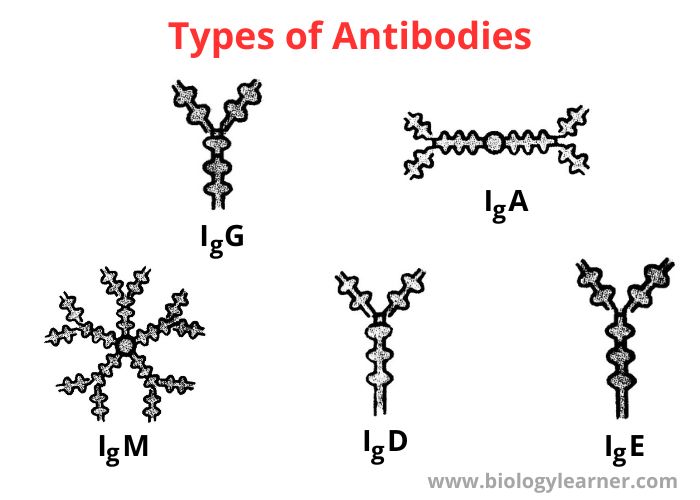
Immunoglobulin G (IgG)
- Immunoglobulin G is the most abundant antibody isotype found in blood serum. It comprises 80% of total serum immunoglobulin.
- It has a monomeric structure (single unit) and composed of two heavy chains and two light chains.
- The molecular weight is about 150000 Da.
- IgG is the only antibody to be transported to the placenta through the fetus and provides considerable immune protection in newborns.
- It protects against bacteria and viruses by enhancing the process of phagocytosis, neutralizing toxins, and triggering complement protein systems.
Immunoglobulin M (IgM)
- Immunoglobulin M or IgM is the first antibody produced in response to an infection by plasma cells.
- It is also the largest antibody and found in pentameric form, in which five monomer units are held together by disulfide bonds.
- There is a J (joining) chain stabilized the whole structure.
- IgM constitutes about 5–10% of the total antibody content in the serum.
- It has a molecular weight of about 970000 Da.
- IgM is involved in opsonization, and agglutination and helps in immune system.
Immunoglobulin A (IgA)
- IgA constitutes 10–15% of antibodies in the serum.
- It is usually found in breast milk, saliva, tears, mucus of the bronchial genitourinary and digestive tracts.
- It exists primarily as a monomer in serum, but polymeric forms are also seen. Both forms have a J-chain polypeptide.
- The molecular weight of IgA is about 160000 Da.
- IgA provides localized protection in the mucosal surfaces such as the intestine, nose, and lungs, etc., against bacteria and viruses.
Immunoglobulin D (IgD)
- IgD comprises less than 1% of the total antibody content in serum.
- It is found as a monomer on the surface of the B cells and acts as antigen receptors.
- It has a molecular weight of about 175000 Da.
- IgD plays a role in the induction of antibody production and is involved in humoral immune responses by regulating B cell selection.
Immunoglobulin E (IgE)
- IgE is found in the least amount in the blood (only 0.1% of all antibodies).
- It is produced by plasma cells and is present as a monomer.
- The molecular weight of IgE is about 190000 Da.
- IgE is involved in allergic and hypersensitivity reactions and provides protection against parasitic worms.
Table: Antibodies, isotypes, Structure, and Function
| Antibody isotype | Subclasses | Structure | Molecular weight | Percentage of total serum antibody | Location | Functions |
| IgG | 4 (IgG1, IgG2, IgG3, IgG4) | Monomer | 150000 Da | 80% | Blood, lymph, intestine | Complement activation |
| IgM | 1 (IgM) | Pentamer with J-chain | 970000 Da | 5-10% | Blood, lymph, B cell surface (as monomer) | Complement activation |
| IgA | 2 (IgA1, IgA2) | Monomer and diamer (with secretory component and J-chain) | 160000 Da | 10-15% | Secretions (saliva, tears, mucus, colostrum) | Localized protection in external secretions |
| IgD | – | Monomer | 175000 Da | 0.2% | B cell surface, blood, lymph | Antigen recognition by B cells |
| IgE | 1 (IgE) | Monomer | 190000 Da | less than 0.1% | Bound to mast and basophill cells throughout body | Involved in allergic reactions |
